PROMO!
First order? Get 10% OFF with this code: 1storder
Written by
LL-37 is an antimicrobial peptide central to the body’s innate immune defense and a key focus in infection research. Studies show that LL-37 peptide can help defend against many bacteria, fungi, and viruses by disrupting microbial membranes and signaling immune cells to act.
At Peptide Works, we see growing demand for LL-37 peptide in laboratory studies as scientists explore its antimicrobial and immune-modulating effects. Research is also highlighting Thymosin Alpha-1 for its potential to support immunity and infection control.
Understanding LL-37’s direct antimicrobial action provides only half the picture. The peptide’s ability to orchestrate immune responses reveals why it remains effective even against rapidly evolving pathogens.
Explore LL-37 peptide from Peptide Works, an antimicrobial peptide that disrupts pathogens and modulates immune defense.
LL-37 peptide does more than break germ walls, it steers immune cells. Once it latches onto a pathogen, LL-37 activates immune cells through receptors including FPR2 and CXCR2 on nearby neutrophils and macrophages.
The bond moves these cells toward the infection site and triggers IL-8 release while restraining excess TNF-α.The cascade also primes dendritic cells to present antigens faster, linking quick innate action to later adaptive control. This two-step defense explains why LL-37 is studied in diverse infection models today.
While LL-37’s signaling capacity explains how immune cells locate threats, the mechanisms neutrophils use to eliminate pathogens once they arrive deserve deeper examination. These front-line defenders employ LL-37 peptide in surprisingly sophisticated ways.
Discover Thymosin Alpha-1 from Peptide Works, a thymic peptide that activates TLR-9, balances cytokines, and strengthens adaptive immunity.
Neutrophils store LL-37 peptide in small granules, ready to act when bacteria appear. When they find bacteria, they release LL-37 into the nearby area. The peptide damages bacterial membranes and helps neutrophils make more germ-killing chemicals. It also strengthens neutrophil extracellular traps (NETs), which are DNA webs that catch microbes and stop toxins.
LL-37 also sends signals through FPR2 and CXCR2 receptors. These signals call more neutrophils to the infection site. By breaking bacteria, reinforcing traps, and bringing reinforcements, neutrophils clear infections faster and stop them from spreading.
FPR2 is key in this process. It helps LL-37 guide neutrophils to act precisely. By combining direct microbial killing with immune signaling, LL-37 shows how the body’s innate defenses can work with accuracy and efficiency.
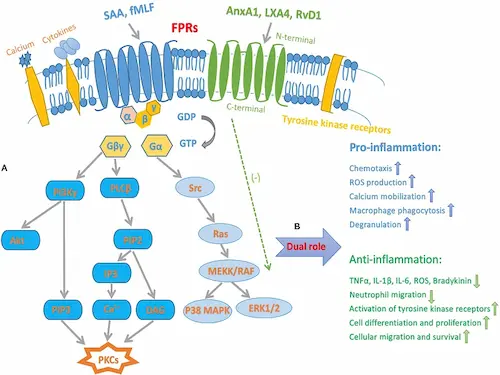
FPR2, short for formyl-peptide receptor 2, is a seven-pass GPCR that acts like a molecular GPS on neutrophils, macrophages, and dendritic cells. LL-37 peptide fits this receptor’s pocket, flipping it into “go” mode.
The signal pulls immune cells up the chemotactic gradient, sparks a measured burst of reactive oxygen species, and prompts IL-8 release while damping runaway TNF-α. Minutes later, the same pathway shifts to pro-resolving ligands, helping shut inflammation down. Because FPR2 drives this attack-and-resolve switch, many labs track LL-37–FPR2 activity when testing add-on peptides such as thymosin alpha-1 for extended host defense.
FPR2 activation consistently triggers reactive oxygen species production a process critical to LL-37’s killing mechanism. Yet many researchers question whether this oxidative burst truly enhances pathogen clearance or poses risks to surrounding tissue.
Yes. When immune cells bind LL-37 peptide through FPR2, they turn on an enzyme that produces reactive oxygen species (ROS) inside the phagosome. These ROS puncture microbial membranes, disable enzymes, and make trapped bacteria easier to digest.
This ROS spike is carefully timed, and bursts are tightly regulated to minimize tissue damage. Studies show that neutrophils exposed to both LL-37 peptide and Thymosin Alpha-1 can sustain a balanced oxidative burst longer, enhancing microbial clearance without increasing collateral damage. Blocking LL-37 peptide in lab assays sharply reduces ROS output, confirming the peptide’s role in oxidative killing.
While LL-37 handles the rapid, front-line defense, Thymosin Alpha-1 complements this by supporting adaptive immune responses. This observation highlights a growing area of research exploring how peptide combinations may enhance overall immune function in a coordinated manner.
Thymosin Alpha-1 is a 28-residue thymic peptide that activates TLR-9 inside dendritic cells. That push matures CD4⁺ and CD8⁺ T cells, lifts IFN-γ and IL-12, and smooths cytokine spikes. Tα1 also tempers responses by inducing IL-10 and IDO, preventing immune overactivation. LL-37 peptide delivers the fast strike rupturing microbial membranes and sparking a brief ROS burst while Thymosin Alpha-1 locks in the adaptive immune response, sharpening antibody production and natural-killer activity.
Together the peptides create true immune synergy: quick pathogen kill followed by durable, balanced host defense. This pairing now anchors many infection-control studies seeking safer, multi-phase protection.
Thymosin Alpha-1’s reliance on TLR-9 activation introduces another layer of immune regulation that many researchers overlook. This receptor’s role in bridging innate and adaptive immunity makes it a crucial component in understanding modern peptide-based therapeutics.
TLR-9, a sensor inside immune cells that detects microbial DNA, sits in dendritic cells and macrophages to spot invading bacteria or viruses. When that DNA docks, TLR-9 triggers the MyD88 pathway, flipping on NF-κB and IRF-7. Within minutes type I interferons and IL-12 surge, co-stimulatory markers rise, and nearby T and NK cells snap to attention.
The signal pushes the immune response from innate to adaptive, sharpening antibody and cytotoxic activity while keeping TNF-α in check. Research peptides such as Thymosin Alpha-1 available from Peptide Works for laboratory use tap this same pathway to fine-tune cytokine balance, making TLR-9 a hot target in infection-control studies.
As antibiotic resistance continues mounting globally, the mechanisms we’ve explored from LL-37’s membrane disruption to TLR-9’s adaptive priming point toward a new paradigm in infection control. The convergence of these pathways suggests where the field is heading.
Germ-fighting and immune-boosting peptides especially LL-37 peptide and Thymosin Alpha-1 are getting more interest as options to regular antibiotics. Their two abilities to break pathogen walls and adjust immune signals make them good study tools for new infection control.
Peptide Works will keep supplying high-quality research peptides so scientists can explore new mixes, delivery ways, and combo plans aimed at faster, safer germ removal.
All products discussed are supplied for research purposes only and are not intended for human use.
[1] Ridyard KE, Overhage J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics (Basel). 2021 May 29;10(6):650.
[2] Zhang Z, Cherryholmes G, Chang F, Rose DM, et al. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol. 2009 Nov;39(11):3181-94.
[3] Zheng Y, Niyonsaba F, Ushio H, Nagaoka I,et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007 Dec;157(6):1124-31.
[4] Dominari A, Hathaway Iii D, Pandav K, Matos W, et al. Thymosin alpha 1: A comprehensive review of the literature. World J Virol. 2020 Dec 15;9(5):67-78.
[5] Huang X, Yang Y. Targeting the TLR9-MyD88 pathway in the regulation of adaptive immune responses. Expert Opin Ther Targets. 2010 Aug;14(8):787-96.
ALL CONTENT AND PRODUCT INFORMATION AVAILABLE ON THIS WEBSITE IS FOR EDUCATIONAL PURPOSES ONLY.
DISCLAIMER: These products are intended solely as a research chemical only. This classification allows for their use only for research development and laboratory studies. The information available on our Peptide Works website: https://peptide-works.com/ is provided for educational purposes only. These products are not for human or animal use or consumption in any manner. Handling of these products should be limited to suitably qualified professionals. They are not to be classified as a drug, food, cosmetic, or medicinal product and must not be mislabelled or used as such.
Peptide Works
Related Articles
What are the Best Cognitive Peptides?
Cognitive peptides are short chains of amino acids that researchers are exploring for their potential effects on brain function. They

Achieving increased skin pigmentation without prolonged sun exposure has been demonstrated with Melanotan peptides in research. Two commonly studied options,

Can PTD-DBM Hair Growth Peptide Stop Balding?
Hair thinning and balding affect millions worldwide, often leading to frustration and limited options. This challenge has driven researchers to