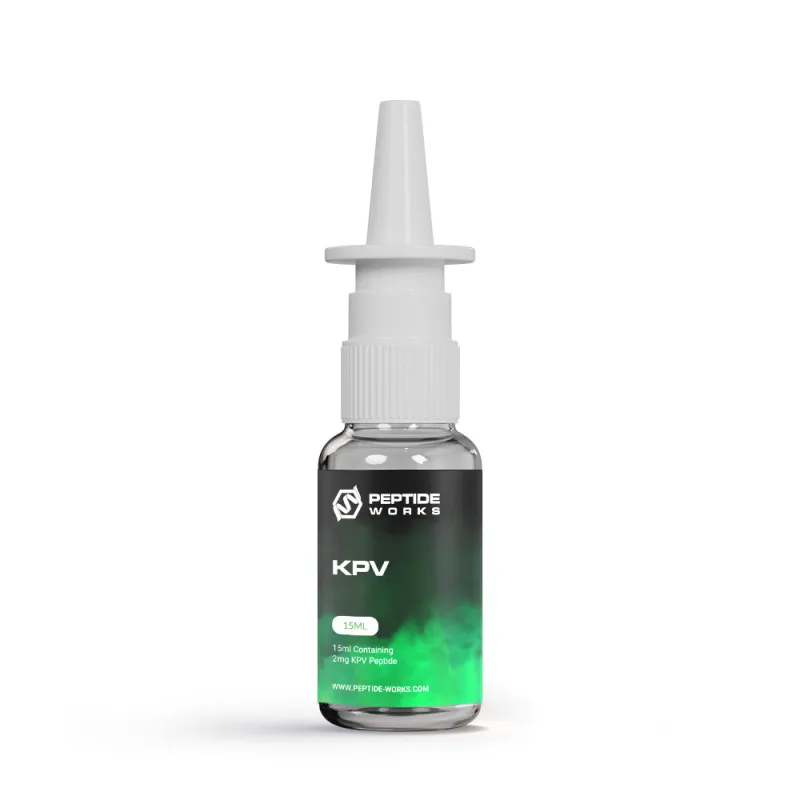
KPV Nasal Spray
The nasal administration of KPV peptide ensures that peptides enter the bloodstream directly, thereby avoiding gastrointestinal degradation and achieving a faster onset. This non-invasive route also enhances compliance during repeated dosing schedules.
Peptide Works offers pre-mixed sprays, eliminating the need for preparation steps. With appropriate storage—cool and refrigerated—the sprays remain stable, effective, and ready for research.
What is KPV?
KPV is a naturally occurring tripeptide composed of lysine, proline, and valine, known for its potent anti-inflammatory and antimicrobial properties. It is derived from the alpha-melanocyte-stimulating hormone (α-MSH) and has been studied for its ability to modulate the immune response and promote healing. KPV works by inhibiting pro-inflammatory cytokines, which are responsible for triggering inflammation in the body. This makes it a promising therapeutic option for conditions such as inflammatory bowel disease (IBD), eczema, psoriasis, and other inflammatory disorders.
In addition to its anti-inflammatory effects, KPV has shown antimicrobial activity, helping to combat harmful bacteria and support skin health. It is often used in topical formulations to aid in wound healing and reduce redness or irritation. With its dual action of lowering inflammation and fighting infections, KPV is gaining attention as a versatile peptide for both medical and cosmetic applications.
Learn about the advantages of KPV Peptide.
Buy KPV Nasal Spray from Peptide Works, a reliable supplier of high purity nasal spray peptides online.
ALL CONTENT AND PRODUCT INFORMATION AVAILABLE ON THIS WEBSITE IS FOR EDUCATIONAL PURPOSES ONLY.
DISCLAIMER: These products are intended solely as a research chemical only. This classification allows for their use only for research development and laboratory studies. The information available on our Peptide Works website is provided for educational purposes only. These products are not for human or animal use or consumption in any manner. Handling of these products should be limited to suitably qualified professionals. They are not to be classified as a drug, food, cosmetic, or medicinal product and must not be mislabelled or used as such.