PROMO!
First order? Get 10% OFF with this code: 1storder
Written by
Ampk activation acts like a cellular energy sensor. It helps control blood glucose levels when diabetes strikes. This protein kinase improves how body uses insulin.
Better insulin sensitivity means glucose uptake works more smoothly in muscle cells. The ampk activity also boosts fatty acid oxidation. This process helps burn fat for energy instead of storing it.
Mitochondrial function gets stronger too. Energy homeostasis becomes more balanced. Peptide Works supplies research-grade Ampk and MOTS-C peptides for scientific studies. Recent research on ARA-290 also highlights its ability to reduce inflammatory stress in metabolic tissues, supporting smoother glucose regulation alongside AMPK-driven pathways.
These compounds show promising results in laboratory settings. The beneficial effects on metabolic control continue to grow.
Explore AMPK Peptide from Peptide Works, a metabolic activator that supports better insulin sensitivity and fat oxidation for improved blood sugar control.
Better insulin sensitivity means your cells respond faster to insulin signals. When ampk activation improves this process, glucose transporters move more easily to cell membranes. This allows glucose uptake to happen without struggle. Your skeletal muscle cells become more efficient at pulling sugar from blood.
The protein kinase helps open cellular pathways that were blocked before. Fatty acid oxidation increases while glucose production slows down. Your body stops making excess sugar when it doesn’t need it.
Cell membranes become more receptive to insulin’s messages. This creates better energy balance throughout your system. Blood sugar levels stay more stable instead of spiking wildly. The beneficial effects happen at the cellular level first.
Check out ARA-290 from Peptide Works, a tissue-protective peptide that reduces inflammatory stress, supports nerve fiber repair, and enhances metabolic function.
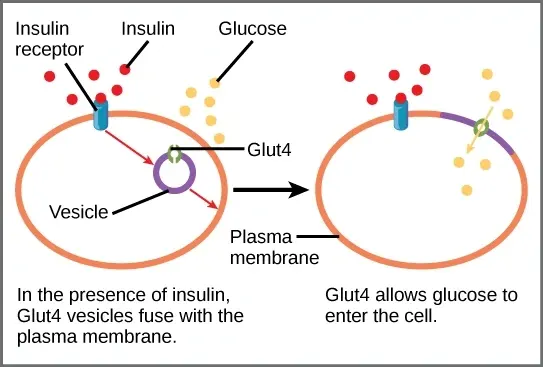
Glucose transporters work like cellular doorways that let sugar enter your cells. GLUT4 transporters sit inside muscle cells waiting for signals. When ampk activation happens, these transporters move to the cell surface quickly. They create pathways for glucose uptake to occur smoothly.
Your skeletal muscle cells contain the most GLUT4 transporters. This makes them powerful glucose-absorbing tissues. The protein kinase helps these transporters relocate faster than normal. Each transporter acts like a cellular energy sensor detecting sugar levels.
GLUT1 transporters handle basic glucose needs in other tissues. Together, they maintain energy homeostasis throughout your body. Mitochondrial function improves when glucose flows properly into cells.
Activation of AMPK triggers signaling pathways that move GLUT4 vesicles toward membranes. Upstream AMPK kinase phosphorylates proteins at the activation loop in vesicles carrying GLUT4.
This conformational change opens binding sites on motor proteins. These proteins then guide vesicles to the cell surface. Activators of AMPK speed this process.
Once on the surface, GLUT4 transporters enable a sudden burst of glucose uptake. This transfer strengthens energy balance during high energy demand.
Fatty acid oxidation may increase as glucose flows into cells. Overall, this mechanism refines blood sugar control without extra insulin.
Ampk Activation flips a structural switch inside the kinase domain of trafficking proteins. This rapid twist drags the catalytic domain toward a fresh binding site that grips vesicle motors.
Once locked, the vesicle’s surface bulges and exposes docking tags that pull GLUT4 to the outer membrane. Small molecule activators can spark the same motion during peak energy demand.
In parallel, the MOTS-C peptide nudges key transcription factors that keep the shift stable. The combined action speeds sugar intake, limits lipid build-up, and guides glucose into cells before levels can spike.
The kinase domain acts like a molecular switch inside cells. When atp levels drop, this domain changes shape and grabs adenosine monophosphate molecules. This triggers kinase activity that sends signals to muscle cells.
The domain then activates protein kinase c pathways that boost sugar absorption. Oxidative stress gets reduced as cells start using glucose more efficiently. The Ampk peptide enhances this domain’s response time.
MOTS-C peptide works alongside by supporting amino acids transport to the same cellular regions. Together, they create a metabolic checkpoint that prevents sugar buildup. This process happens in intact cells during normal cellular function. The domain’s activity directly impacts blood glucose management.

The metabolic checkpoint acts like a cellular traffic light for sugar flow. AMPK Activation triggers this checkpoint to open pathways that let glucose enter cells faster. ARA-290 complements this response by stabilizing stressed tissues and calming cytokine activity, helping the checkpoint operate more efficiently during metabolic strain.
When energy demand rises, this process prevents sugar from flooding tissues and causing metabolic syndrome. The checkpoint stops cell cycle arrest by maintaining steady energy balance.
It monitors energy status constantly and adjusts glucose flow accordingly. This important role keeps blood sugar stable during stress or exercise capacity changes. The checkpoint also blocks excess sugar from turning into fat storage.
Bateman domains help coordinate these protective signals. This system prevents glucose overload that damages organs over time.
Discover MOTS-c Peptide from Peptide Works, a mitochondrial peptide that enhances glucose metabolism and cellular energy balance to aid in diabetes management.
Energy balance sets the pace for glucose flow in diabetes. When AMPK Activation senses low ATP, it shifts fuel use. It tilts lipid metabolism toward burning fat instead of storing it.
At the same time, it calms glycogen synthase, slowing new sugar storage in the liver. This fine tuning stops sharp sugar swings that strain organs. Healthy balance also spares the endoplasmic reticulum from overload by matching intake with need.
In muscle and adipose tissues, this harmony supports steady energy and helps manage daily diabetic challenges seen during routine physical activity.
AMPK Activation opens new pathways for diabetes-related laboratory investigations. Recent study explore how cellular mechanisms respond to targeted compounds in controlled environments.
Advanced research may reveal deeper connections between energy regulation and glucose metabolism at the molecular level. Peptide Works provides researchers with quality compounds needed for these scientific explorations.
Future studies could uncover novel therapeutic targets within cellular signaling pathways. Laboratory findings may lead to breakthrough discoveries in metabolic regulation mechanisms.
This research direction shows promise for advancing our understanding of diabetes at the cellular level. Scientific progress in this field continues to expand possibilities for future therapeutic development.
All products discussed are supplied for research purposes only and are not intended for human use.
References:
[1] Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014 Jun 24;7:241-53.
[2] Kakoti BB, Alom S, Deka K, Halder RK. AMPK pathway: an emerging target to control diabetes mellitus and its related complications. J Diabetes Metab Disord. 2024 Apr 18;23(1):441-459.
[3] Veiseh O, Kievit FM, Ellenbogen RG, Zhang M. Cancer cell invasion: treatment and monitoring opportunities in nanomedicine. Adv Drug Deliv Rev. 2011 Jul 18;63(8):582-96.
[4] Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell. 2017 Jun 15;66(6):789-800.
[5] Yang Q, Zhao J, Chen D, Wang Y. E3 ubiquitin ligases: styles, structures and functions. Mol Biomed. 2021 Jul 30;2(1):23.
[6] Jung SM, Sanchez-Gurmaches J, Guertin DA. Brown Adipogenesis Tissue Development and Metabolism. Handb Exp Pharmacol. 2019;251:3-36.
ALL CONTENT AND PRODUCT INFORMATION AVAILABLE ON THIS WEBSITE IS FOR EDUCATIONAL PURPOSES ONLY.
DISCLAIMER: These products are intended solely as a research chemical only. This classification allows for their use only for research development and laboratory studies. The information available on our Peptide Works website: https://peptide-works.com/ is provided for educational purposes only. These products are not for human or animal use or consumption in any manner. Handling of these products should be limited to suitably qualified professionals. They are not to be classified as a drug, food, cosmetic, or medicinal product and must not be mislabelled or used as such.
Peptide Works
Related Articles
What are the Best Cognitive Peptides?
Cognitive peptides are short chains of amino acids that researchers are exploring for their potential effects on brain function. They

Achieving increased skin pigmentation without prolonged sun exposure has been demonstrated with Melanotan peptides in research. Two commonly studied options,

Can PTD-DBM Hair Growth Peptide Stop Balding?
Hair thinning and balding affect millions worldwide, often leading to frustration and limited options. This challenge has driven researchers to